saturated cyclic hydrocarbon|is cyclohexane saturated or unsaturated : Tuguegarao Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. Cycloalkanes are also saturated, meaning . Resultado da 18 de set. de 2023 · Confira o resultado da Lotofácil 2907 no site Foco em Loterias, veja os ganhadores e números sorteados da Lotofácil 2907 que .
0 · saturated hydrocarbon examples
1 · naming of cyclic compounds
2 · naming cycloalkanes with substituents
3 · naming cycloalkanes rings as substituents
4 · naming compounds with cycloalkyl substituents
5 · is cyclopropane saturated or unsaturated
6 · is cyclohexane saturated or unsaturated
7 · cyclic hydrocarbon naming
8 · More
6 dias atrás · Resultado do jogo do bicho do Rio de Janeiro atualizado de hoje 11:00 Horas, Resultado das 14:00 Horas PT RJ e Coruja das 21:00 Horas, além do Resultado da Federal do Rio de Janeiro - RJ. Deu no Poste hoje, todos os dias atualizados. Aproveite para conferir nossos Palpites do dia Resultado Fácil diariamente, possuímos inúmeras .
saturated cyclic hydrocarbon*******A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes, cycloalkanes are saturated compounds.
Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the .A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a .
Cycloalkanes are cyclic hydrocarbons, meaning that the carbons of the molecule are arranged in the form of a ring. Cycloalkanes are also saturated, meaning . A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon .Saturated cyclic hydrocarbons are called cycloalkanes, or alicyclic compounds (aliphatic cyclic). Because cycloalkanes consist of rings of −CH 2 −units, they have the general . A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon .
Alkanes are saturated hydrocarbons, cyclic or acyclic in nature. Small chain hydrocarbons are the main constituents of petrochemical and natural gas [17,18]. A small portion of .The simplest of the saturated hydrocarbons, or cycloalkanes, is cyclopropane, C 3 H 6, the molecules of which are made up of three carbon atoms to each of which two hydrogen . Cyclic hydrocarbons, also referred to as ring structures or cyclic compounds, are extremely versatile and useful compounds that are involved in a variety .
Results of this study reveal contrasting binding nature of the acyclic and cyclic hydrocarbons towards CNSs. While the saturated molecules show stronger binding affinity in acyclic hydrocarbons; the unsaturated .
As indicated by its name, a cycle hydrocarbon represents a ring structure comprised of carbon atoms attached by covalent bonds to each other. Cyclic refers to the ring, or circular, shape of the . Cycloalkanes are saturated hydrocarbons that contain only one ring. A cycloalkane is a cyclic hydrocarbon with single carbon-carbon bonds on all of its carbon atoms. They have two fewer hydrogen atoms than an alkane with the same number of carbon atoms, with a general formula of CnH2n (n is an integer greater than 2).saturated cyclic hydrocarbon is cyclohexane saturated or unsaturated The general formula for saturated hydrocarbons is C n H 2n +2 (assuming non-cyclic structures) as shown in hexane (C 6 H 14) below. Cycloalkanes are hydrocarbons containing one or more carbon rings to which hydrogen atoms are attached. The general formula for a cyclic hydrocarbon containing one ring is C n H 2n as shown .
A saturated hydrocarbon is a hydrocarbon that is made of single carbon-carbon bonds. Such hydrocarbons are characterized by having the maximum number of H atoms per C atom.
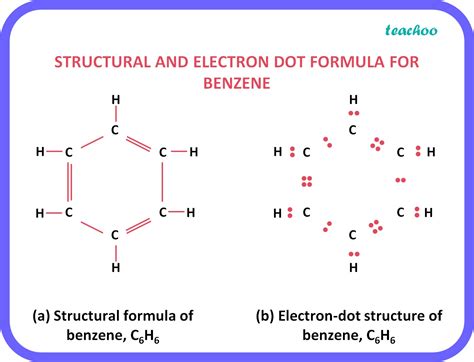
The general formula for saturated hydrocarbons is C n H 2n +2 (assuming non-cyclic structures) as shown in hexane (C 6 H 14) below. Cycloalkanes are hydrocarbons containing one or more carbon rings to which hydrogen atoms are attached. The general formula for a cyclic hydrocarbon containing one ring is C n H 2n as shown in .is cyclohexane saturated or unsaturated A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes, cycloalkanes are saturated compounds. Cycloalkanes have the general formula CnH2n C n H 2 n. The simplest cycloalkane is .
Alkanes. Alkanes, or saturated hydrocarbons, contain only single covalent bonds between carbon atoms.Each of the carbon atoms in an alkane has sp 3 hybrid orbitals and is bonded to four other atoms, each of which is either carbon or hydrogen. The Lewis structures and models of methane, ethane, and pentane are illustrated in Figure \(\PageIndex{1}\).
3.7: Saturated Hydrocarbons. Approximately one-third of the compounds produced industrially are organic compounds. All living organisms are composed of organic compounds, as are most foods, medicines, clothing fibers, and plastics. The detection of organic compounds is useful in many fields. In one recently developed application, .The formula for acyclic saturated hydrocarbons (i.e., alkanes) is C n H 2n+2.: 623 The most general form of saturated hydrocarbons, (whether linear or branched species, and whether with without one or more rings) is C n H 2n+2(1-r), where r is the number of rings. Those with exactly one ring are the cycloalkanes. Cyclic Hydrocarbons. A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes, cycloalkanes are saturated compounds. cycloalkanes: Cyclic saturated hydrocarbons with a general formula of CnH(2n). Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. functional groups: An atom or groups of atoms that substitute for a hydrogen atom in an organic compound, giving the compound unique chemical properties and determining its .cycloalkanes: Cyclic saturated hydrocarbons with a general formula of CnH(2n). Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. functional groups: An atom or groups of atoms that substitute for a hydrogen atom in an organic compound, giving the compound unique chemical properties and determining its reactivity.The general formula for saturated hydrocarbons is \(C_nH_{2n+2}\) (assuming non-cyclic structures). Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched species.The simplest alkanes have their C atoms bonded in a straight chain; these are called normal alkanes. They are named according to the .
Alkanes are saturated hydrocarbons, cyclic or acyclic in nature. Small chain hydrocarbons are the main constituents of petrochemical and natural gas [17,18]. A small portion of these alkanes (C 9 –C 19) are useful as fuels, but rest of the highly abundant alkanes in nature remain largely unproductive [5]. Small chain alkanes such as propane . cycloalkanes: Cyclic saturated hydrocarbons with a general formula of CnH(2n). Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. functional groups: An atom or groups of atoms that substitute for a hydrogen atom in an organic compound, giving the compound unique chemical properties and determining its .
cycloalkanes: Cyclic saturated hydrocarbons with a general formula of CnH(2n). Cycloalkanes are alkanes with carbon atoms attached in the form of a closed ring. functional groups: An atom or groups of atoms that .
The general formula for saturated hydrocarbons is \(C_nH_{2n+2}\) (assuming non-cyclic structures). Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched .Alkanes are saturated hydrocarbons, cyclic or acyclic in nature. Small chain hydrocarbons are the main constituents of petrochemical and natural gas [17,18]. A small portion of these alkanes (C 9 –C 19) are useful as fuels, but rest of the highly abundant alkanes in nature remain largely unproductive [5]. Small chain alkanes such as propane .A is a saturated cyclic hydrocarbon and B and C are unsaturated cyclic hydrocarbons. Reason — A is having all the single bonds hence it is a saturated hydrocarbon whereas B and C have double bonds so they are unsaturated cyclic hydrocarbons. Answered By. 19 Likes. Related Questions. Select washing soda from the following :saturated cyclic hydrocarbon A cyclic hydrocarbon is a hydrocarbon in which the carbon chain joins to itself in a ring. A cycloalkane is a cyclic hydrocarbon in which all of the carbon-carbon bonds are single bonds. Like other alkanes, cycloalkanes are saturated compounds. Cycloalkanes have the general formula of C n H 2n. The simplest cycloalkane is .

The classifications for hydrocarbons are summarized below. Saturated hydrocarbons (alkanes) are the simplest of the hydrocarbon species. They are composed entirely of single bonds and are saturated with hydrogen. The general formula for saturated hydrocarbons is C n H 2n+2 (assuming non-cyclic structures). Saturated .Cycloalkane. In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. [1] In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containing a single ring (possibly with side chains ), and all of the carbon-carbon bonds are .Saturated hydrocarbons . Cyclic esters, referred to as lactones, are realized in numerous natural products. Remarkable examples are the so-called macrolides, cyclic biomolecules with rings of usually 14–16 atoms (maximum variation of ring size 6–62) frequently used as antibiotics. Cyclic amides, the so-called lactams, are of lesser .
Organic Chemistry. Dr. James G. Speight, in Environmental Organic Chemistry for Engineers, 2017 2.1.2 Cycloaliphatic Hydrocarbons. Cycloaliphatic hydrocarbons (also called cycloalkanes, alicyclic hydrocarbons, naphthenes) are saturated hydrocarbons containing one or more rings, each of which may have one or more paraffin (alkyl) side .Alkane. In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings ), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. [1] Alkanes have the general chemical formula CnH2n+2.
By far the most common reaction of hydrocarbons is combustion, which is the combination of a hydrocarbon with O 2 to make CO 2 and H 2 O. The combustion of hydrocarbons is accompanied by a release of energy and is a primary source of energy production in our society (Figure 16.2.2 16.2. 2 - Combustion).
1. 138 votes, 31 comments. 3.1K subscribers in the bellekaffer community. Brazilian Youtuber And Singer Belle Kaffer's Reddit Community.
saturated cyclic hydrocarbon|is cyclohexane saturated or unsaturated